
The cookie is used to store the user consent for the cookies in the category "Analytics". This cookie is set by GDPR Cookie Consent plugin. These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. The bond angles of Carbon with Hydrogen and Chlorine atoms are 109.5 degrees. In the Lewis structure of CH3Cl, Carbon is at the central position and all the other atoms around it.
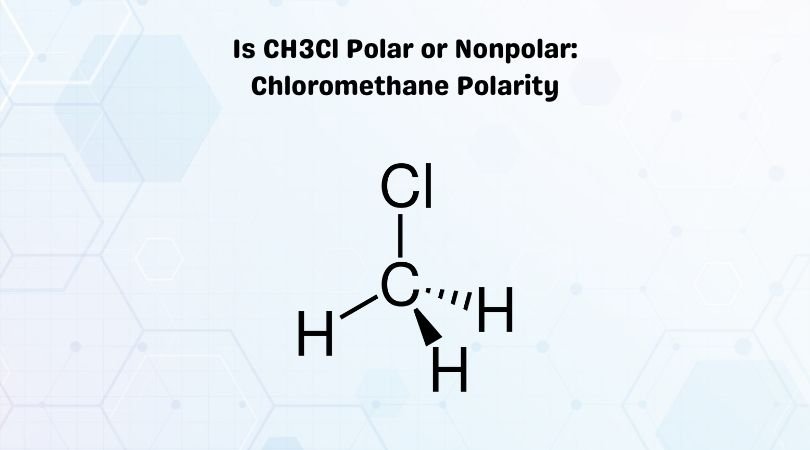
It has 14 valence electrons, and all of them participate in forming bonds. To summarize, we can say the following for a single molecule of Chloromethane. How many valence electrons are in a CH3Cl molecule? Molecular Geometry of CH3Cl The CH3Cl is a Penta atomic molecule with a bond angle of 109.5° which gives the molecule a bent shape. Which is the bent shape of CH3Cl molecule? If you click on the example molecules (where it says 3D view) below you’ll get a better understanding of why some molecules are polar and some not. You need to consider the molecule in 3D (three dimensions).
#Ch3cl polarity how to
To really understand how to do this, the Lewis structure is only the first step. How to tell if a molecule is polar or non polar? The diagram is drawn to determine how the valence electrons of different atoms participate in the bond formation to form a molecule. Lewis Structure of Chloromethane (CH3Cl) The Lewis structure is a pictorial representation showing the electrons present in the valence shell in an atom. How does the Lewis structure of CH3Cl work? Another non polar molecule shown below is boron trifluoride, BF3. Notice that a tetrahedral molecule such as CCl4 is nonpolar Figure (4.12. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical – bonded to the same element with no unshared pairs of electrons. Furthermore, it has a linear molecular structure and the nature of C-H bonds is non-polar covalent. Is C2H2 polar or nonpolar molecule?Ĭ2H2 is nonpolar in nature because the electronegativity difference between Carbon and Hydrogen is 0.35, which is less than the minimum required 0.4. Methane is non-polar as the difference in electronegativities between carbon and hydrogen is not great enough to form a polarized chemical bond. Methane (CH4) is a non-polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms. The C-Cl covalent bond shows unequal electronegativity because Cl is more electronegative than carbon causing a separation in charges that results in a net dipole. So, Is CH3Cl polar or non-polar? Yes, Methyl chloride (CH3Cl) or Chloromethane is a polar molecule.
Lewis Structures and the Shapes of Molecules Which Vsepr shapes are polar and nonpolar? With CH3Cl however, You have 3 C-H bond at tetrahedral angles but the C-Cl bond, more polar than the C-H bond, occupies the fourth apex of the tetrahedron and the CH3Cl is not canceled out as in CH4 so CH3Cl is polar. Which molecule is polar CH3Cl and CH4?ĬH4 is tetrahedral in shape so each of the polarities of each of the C-H bonds cancel each other. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero. So, is CH4 polar or nonpolar? CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. How do you know if CH4 is polar or nonpolar? Because the C-Cl bond is polar, the CH3Cl has a net dipole, so CH3Cl is polar. Since the H is between B and C in terms on electronegativity values, their difference in electronegativity values is so small, the C-H bond is considered nonpolar thus, no dipole arrow is drawn for the C-H bonds.

How do you know if a CH3Cl is polar or nonpolar?


 0 kommentar(er)
0 kommentar(er)
